
According to doctors, if you have had more than one sex partner, you should be tested. If you’re having new partners from one year to the next, you should be tested on a routine basis, even if you’re using safer-sex techniques. If you come down with flu-like symptoms soon after a risky sexual encounter such as unprotected sex or a drug-related episode such as needle sharing, that’s a good time to get tested.
Still according to doctors, in about half of all cases, a person will get an acute infection within a few days of contracting the HIV virus. The tragedy is that doctors may confuse this infection with flu or mononucleosis. The real cause may not be known for years.

After a person contracts the HIV virus, it may take up to three months before he develops the HIV antibodies that the tests pick up. This is what’s known as the “window period.” If you’ve had a risky encounter, you may want to wait three months before getting tested (unless you get signs of a viral infection). In rare cases, it may take up to six months before antibodies develop.
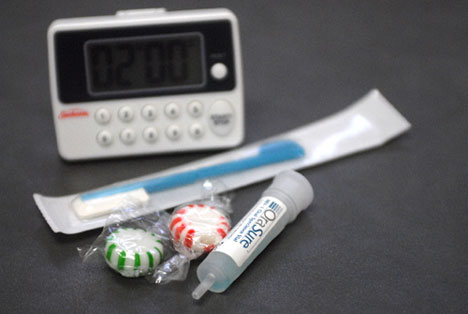
HIV tests come as either blood test or oral test. In some tests, you may get the results in 20 minutes, while with some tests, you’ll be asked to wait several days for your results. With rapid testing, you’ll know right away if you’re negative. If the test comes back positive, however, you may have to wait a few days while a second test is done to confirm the first test.
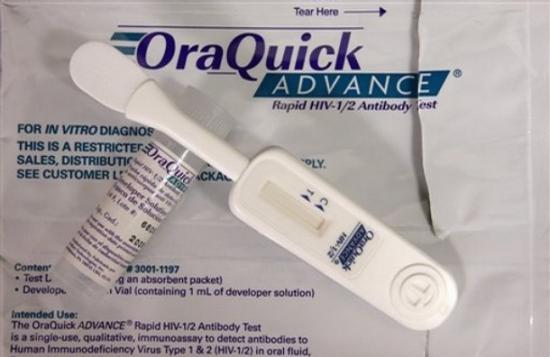
The Centers for Disease Control and Prevention (CDC), a part of the U.S. Department of Health and Human Services, is the primary Federal agency for conducting and supporting public health activities in the United States. It is also where the term AIDS started. The CDC endorses the Rapid HIV Tests currently available in the United States. Rapid HIV tests are simple to use and require little or no specialized equipment. They make it possible to provide test results at the time the test is done. The following are the six rapid HIV tests approved by the U.S. Food and Drug Administration (FDA):
1. OraQuick Rapid HIV-1/2 Antibody Test
2. Reveal G3 Rapid HIV-1 Antibody Test
3. Uni-Gold Recombigen HIV Test
4. Multispot HIV-1/HIV-2 Rapid Test
5. Clearview HIV 1/2 Stat Pak
6. Clearview Complete HIV ½
There is no nationally or internationally accepted criteria for what constitutes a positive result. Standards also vary from lab to lab within the same country or state, and can even differ from day to day at the same lab. As HIV test kit manufacturers acknowledge, “At present there is no recognized standard for establishing the presence or absence of antibodies to HIV-1 and HIV-2 in human blood.”
The HIV Antibody Test is not standardized.

Since 1986, the HIV antibody tests used worldwide continue to carry an alarming disclaimer:
At present, there is no recognized standard for establishing the presence or absence of antibodies to HIV-1 and HIV-2 in human blood.
Rodney Richards, Ph.D. worked on the development of antibody (ELISA) and genetic “viral load” tests for Amgen and holds some related patents. He says, “The diagnosis of being HIV positive is based on arbitrary combinations of tests, none of which are approved for diagnosing HIV. In fact there is no test for HIV. It’s just an illusion.”
Raising issues of informed consent for all persons submitting to HIV antibody testing, the test kits themselves contain disclaimers that doctors rarely, if ever, share with patients. For example, Abbott Laboratories’ ELISA test kit, typically used as a preliminary test, warns:
ELISA testing alone cannot be used to diagnose AIDS.
Confirmation of an ELISA result with a Western Blot test is currently required as a “standard of care.”
Epitope’s Western Blot package insert reads:
Do not use this kit as the sole basis for HIV infection.
This becomes more concerning because the Western Blot is supposed to be a highly accurate test, used to confirm that an ELISA is not a false positive.
The fine print on newer rapid tests expresses similar uncertainty, specifying they are intended only to:
aid in the diagnosis of infection with HIV
These rapid tests do not actually diagnose HIV infection. They also further note that AIDS is merely “thought to be caused by HIV” rather than known to be the cause.
The package insert accompanying viral load tests still declares they are “not intended to be used as a screening test for HIV or as a diagnostic test for confirm the presence of HIV infection (HIV-1/HIV-2 EIA/ELISA, Abbot Laboratories; OraQuick Rapid HIV-1 Antibody Test, Abbot Diagnostics; Amplicor HIV-1 Monitor Test, Roche).
There is no actual test for HIV.

Many people are surprised to learn that there is no such thing as a test for AIDS. The tests popularly referred to as “AIDS tests” do not identify or diagnose AIDS and cannot detect HIV, the virus claimed to cause AIDS. The ELISA and Western Blot tests commonly used to diagnose HIV infection detect only interactions between proteins and antibodies thought to be specific for HIV — they do not detect HIV itself. And contrary to popular belief, newer “viral load” tests do not measure levels of actual virus in the blood.
One reason for the tests’ tremendous inaccuracy is that a variety of viruses, bacteria and other antigens can cause the immune system to make antibodies that also react with HIV. When the antibodies produced in response to these other infections and antigens react with HIV proteins, a positive result is registered. Many antibodies found in normal, healthy, HIV-free people can cause a positive reading on HIV antibody tests. Since the antibody production generated by a number of common viral infections can continue for years after the immune system has defeated a virus — and even for an entire lifetime — people never exposed to HIV can have consistent false positive reactions on HIV tests for years or for their entire lives.
The accuracy of an antibody test can be established only by verifying that positive results are found in people who actually have the virus. This standard for determining accuracy was not met in 1984 when the HIV antibody test was first created. Instead, to this day, positive ELISAs are verified by a second antibody test of unknown accuracy, the HIV Western Blot. Since the accuracy for HIV antibody tests has never been properly established, it is not possible to claim that a positive test indicates a current, active HIV infection or even to know what it may indicate. In one study that investigated positive results confirmed by Western Blot, 80 people with two positive ELISAs that were “verified” by a positive Western Blot tested negative on their next Western Blot.
The famous HIV test is inaccurate.
Antibodies produced in response to simple infections like a cold or the flu can cause a positive reaction on an HIV antibody test. A flu shot and other immunizations can also create positive HIV ELISA and Western Blot results. Having or having had herpes or hepatitis may produce a positive test, as can vaccination for hepatitis B. Exposure to microbes such as those that cause tuberculosis and malaria commonly causes false positive results, as do the presence of tapeworms and other parasites.
Conditions such as alcoholism or liver disease and blood that is altered through drug use may elicit the production of antibodies that react on HIV antibody tests. Pregnancy and prior pregnancy can also cause a positive response. The antibodies produced to act against infection with mycobacterium and yeast infections, found in 90% of AIDS patients, cause false positive HIV test results.
In one study, 13% of Amazonian Indians who do not have AIDS and who have no contact with people outside their own tribe tested HIV positive. In another report, 50% of blood samples from healthy dogs reacted positively on HIV antibody tests.
It is important to note that none of the proteins used in HIV antibody tests are particular to HIV, and none of the antigens said to be specific to HIV are found only in persons who test HIV positive. In fact, many people diagnosed HIV positive do not have these “HIV antigens” in their blood.
Newer HIV “viral load” tests do not isolate or measure actual virus. The tests’ manufacturers clearly state that viral load
is not intended to be used as a screening test for HIV or as a diagnostic test to confirm the presence of HIV infection.
In fact, viral load tests have not been approved by the FDA for diagnostic purposes and have not been verified by virus isolation.


The only way to distinguish between real reactions and cross-reactions is to use HIV isolation. All claims of HIV isolation are based on a set of phenomena detected in tissue culture, none of which are isolation and none of which are even specific for retroviruses…We don’t know how many positive tests occur in the absence of HIV infection. There is no specificity of the HIV antibody tests for HIV infection.
Bio/Technology Journal, 11:696-707, 1993
The HIV antibody tests do not detect a virus. They test for any antibodies that react with an assortment of proteins experts claim are specific to HIV. The fact is that an antibody test, even if repeated and found positive a thousand times, does not prove the presence of viral infection.
Val Turner, MD, Continuum magazine, Vol 3 No 5, 1996
Should you bet your life on an HIV test?

All information stated above comes from the decades-long debates on the validity of the HIV hypothesis. Simply google “myth of AIDS” and find out for yourself the many points and issues aimed at disclosing the hoax.
The Human Rumor Virus: The AIDS Lie (Part 1)



